New Delhi, 08 May: Presently, the Defense Research and Development Organization (DRDO) scientists have developed a 2-deoxy-D-glucose (2-DG) drug in collaboration with Dr. Reddy’s Labs during the ongoing pandemic across the country. It has been approved by the Drugs Controller General of India (DCGI) for medical emergency use on serious patients of Covid-19.
Meanwhile, RT-PCR tests of most covid patients have been reported negative after treatment with 2-DG. DRDO states that this drug can be easily produced and made available in the market.
The DRDO was informed on Saturday that after the completion of the clinical trial, now the Drugs Controller General of India (DCGI) on May 1 provided emergency use of this drug as adjuvant therapy for severe Covid-19 patients.
Now it can be easily produced and made available in abundance in the country. DCGI permitted a 2-DG Phase II clinical trial in Covid patients in May 2020. The drug was found safe in trials conducted on patients from May to October 2020 and there was significant improvement in the condition of the patients.
However, one part of the test was done in 6 hospitals and the other part in 11 hospitals in the country. In total, 110 patients were used in the second phase clinical trial. In the same way, there are many more.
Testing has shown that this drug helps in rapid recovery of hospitalized patients and also reduces their oxygen dependence.
According to the DRDO, Covid patients on whom 2-DG was used during the trial endured symptoms of the disease more quickly than the standard of care prescribed. This drug comes in the form of powder, which is dissolved in water and to be given to the patient.
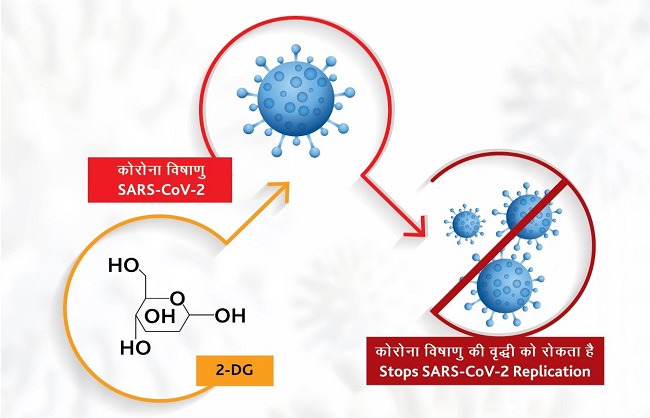
DRDO said that this drug accumulates in virus-infected cells and prevents the virus from moving in the body. DRDO has stated in an official statement that this drug can be used as an adjunct or alternative to ongoing treatment of serious Covid patients. Its purpose is to provide first aid.
DRDO said that during the first wave of the Covid-19 pandemic in April 2020, the organization’s scientists developed the drug by DRDO’s Lab Institute of Nuclear Medicine and Allied Sciences in collaboration with Reddy’s laboratories.
Under the auspices of the Institute of Nuclear Medicine and Allied Sciences (INMAS) and the Council of Industrial Research conducted several experiments of 2-deoxy-D-glucose with the help of Lab Cellular and Molecular Biology (CCMB) of Hyderabad. Testing conducted in the laboratory found it has been suggested that this drug works effectively against severe acute respiratory syndrome coronavirus (SARS-CoV-2) and inhibits its growth.